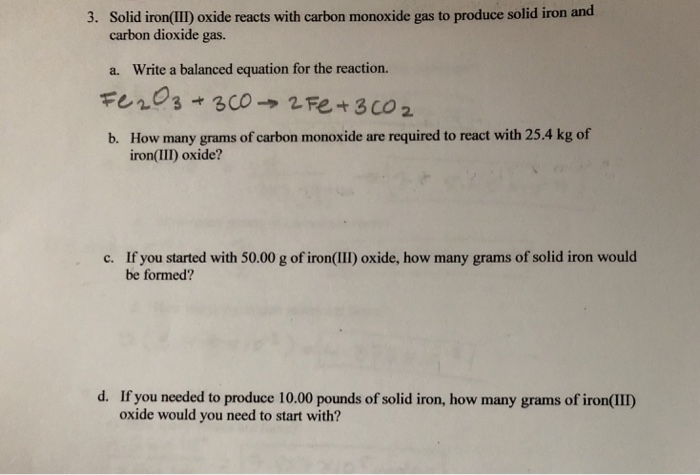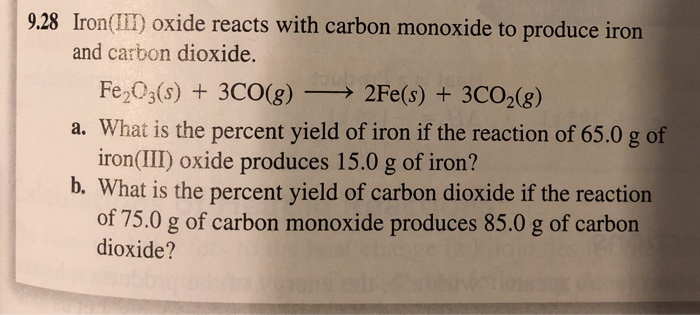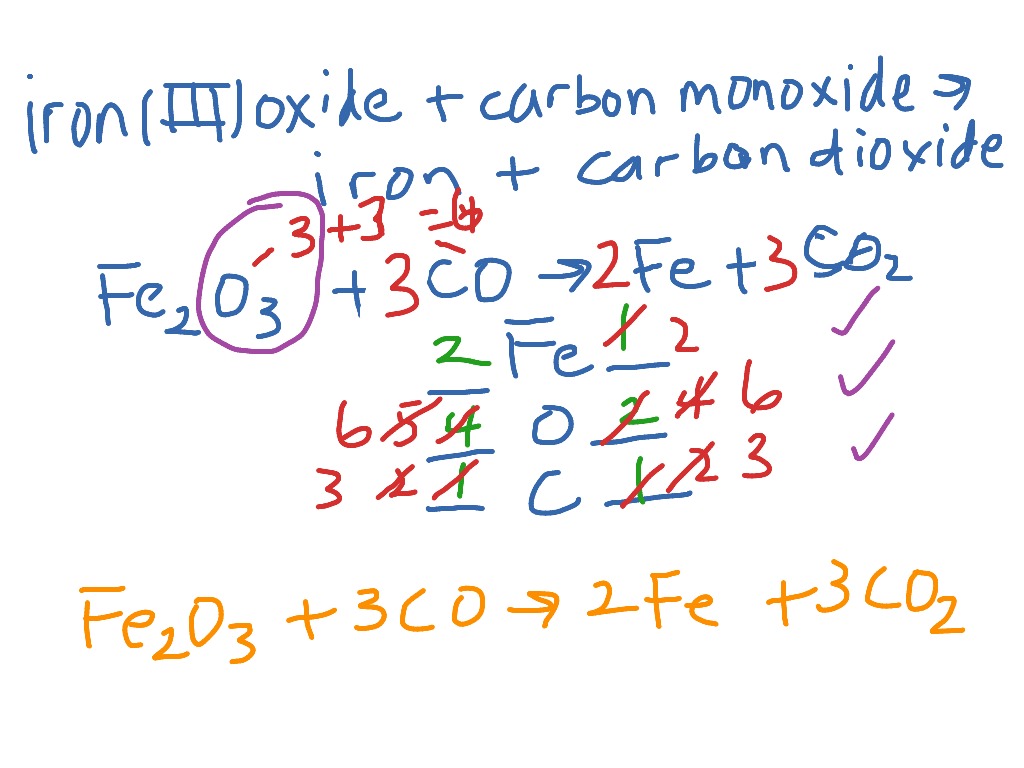Barium and water yields iron (III) chloride. Lead (II) oxide and carbon react to make solid lead and carbon dioxide. - ppt download

Iron is obtained by reducing iron (III) oxide using the gas carbon monoxide. The reaction is Fe2O3 + 3CO - Brainly.com
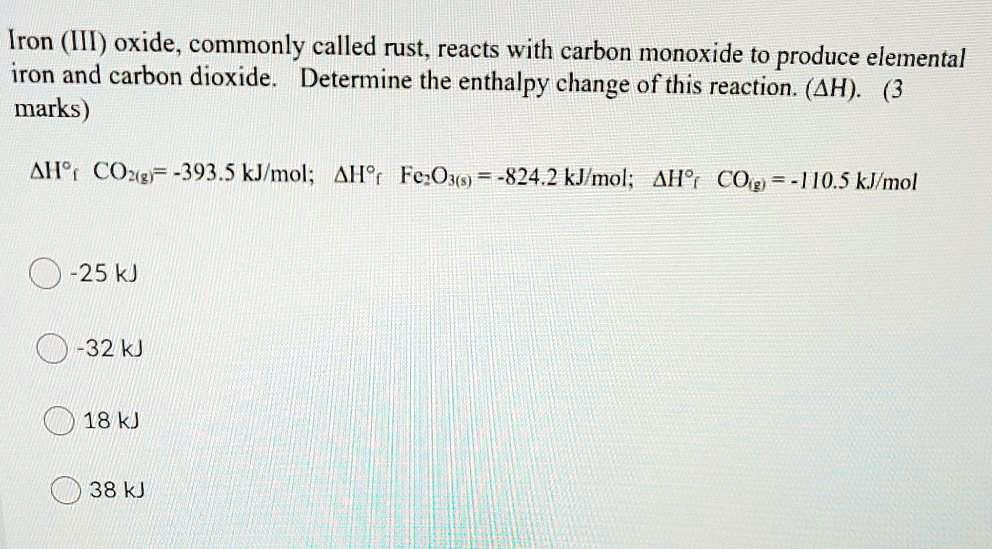
SOLVED: Iron (III) oxide, commonly called rust, reacts with carbon monoxide to produce elemental iron and carbon dioxide. Determine the enthalpy change of this reaction. (ΔH) (3 marks) ΔH CO = -393.5
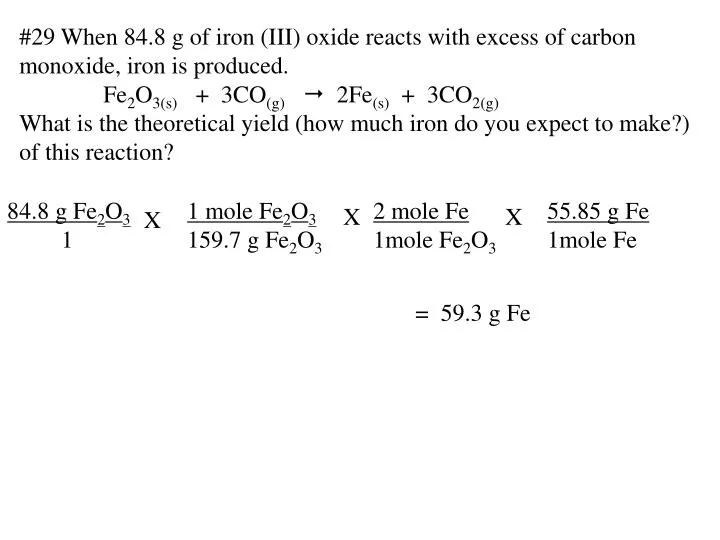
PPT - #29 When 84.8 g of iron (III) oxide reacts with excess of carbon monoxide, iron is produced. PowerPoint Presentation - ID:4448728

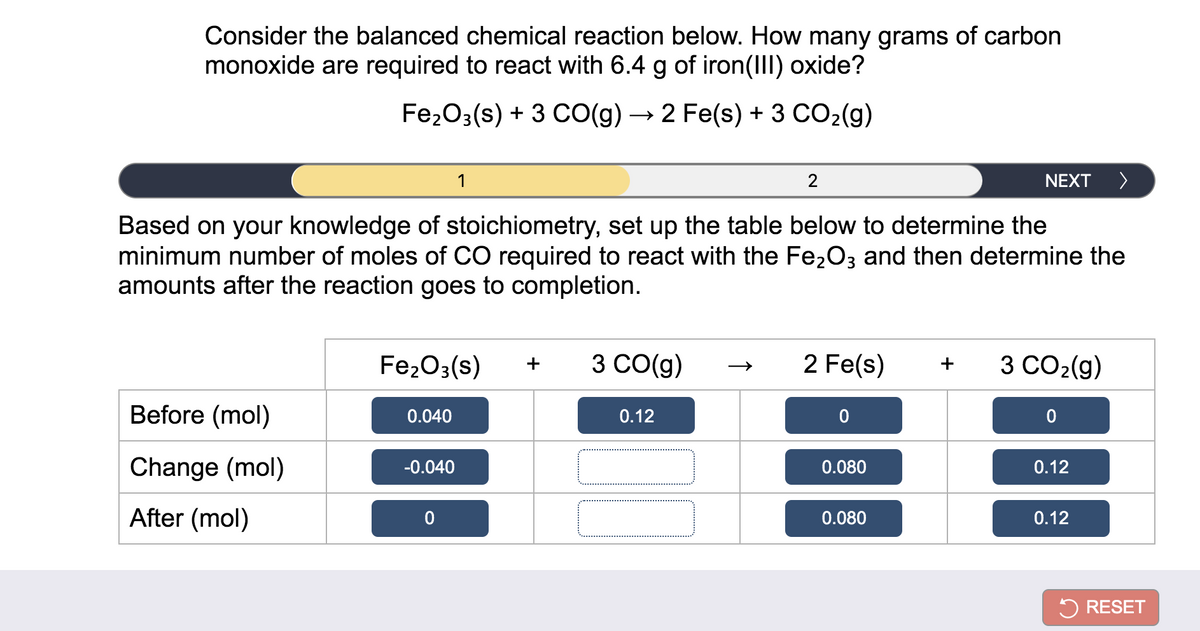
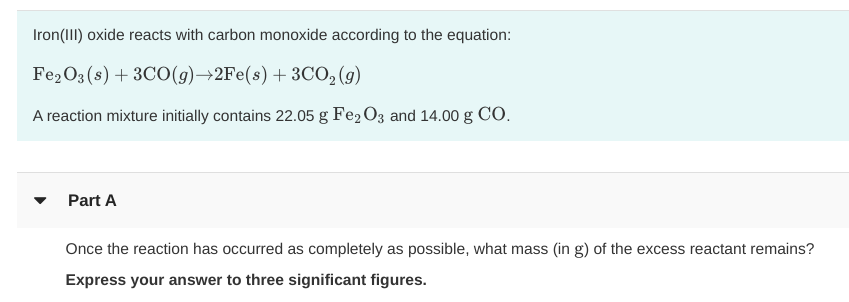
SOLVED:Iron(III) oxide reacts with carbon monoxide according to the equation: Fe2 O3(s)+3 CO(g) ⟶2 Fe(s)+3 CO2(g) A reaction mixture initially contains 22.55 g Fe2 O3 and 14.78 g CO. Once the reaction
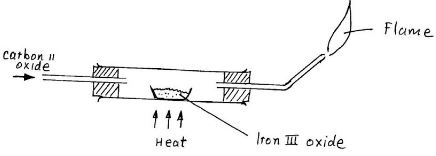
Carbon Oxide gas was passed over heated iron III Oxide as shown in the diagram below - EasyElimu Questions and Answers
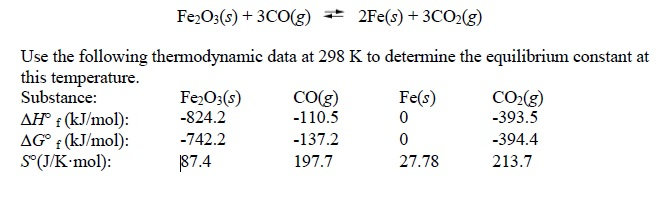
Iron is produced by the reduction of iron (III) oxide using carbon monoxide. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g). How much Fe is produced from 1 kg of Fe2O3? - Quora
28. Magnetite is an iron oxide ore, which reacts with carbon monoxide to give iron metal and carbon dioxide. When a sample of magnetite is allowed to react with sufficient carbon monoxide,

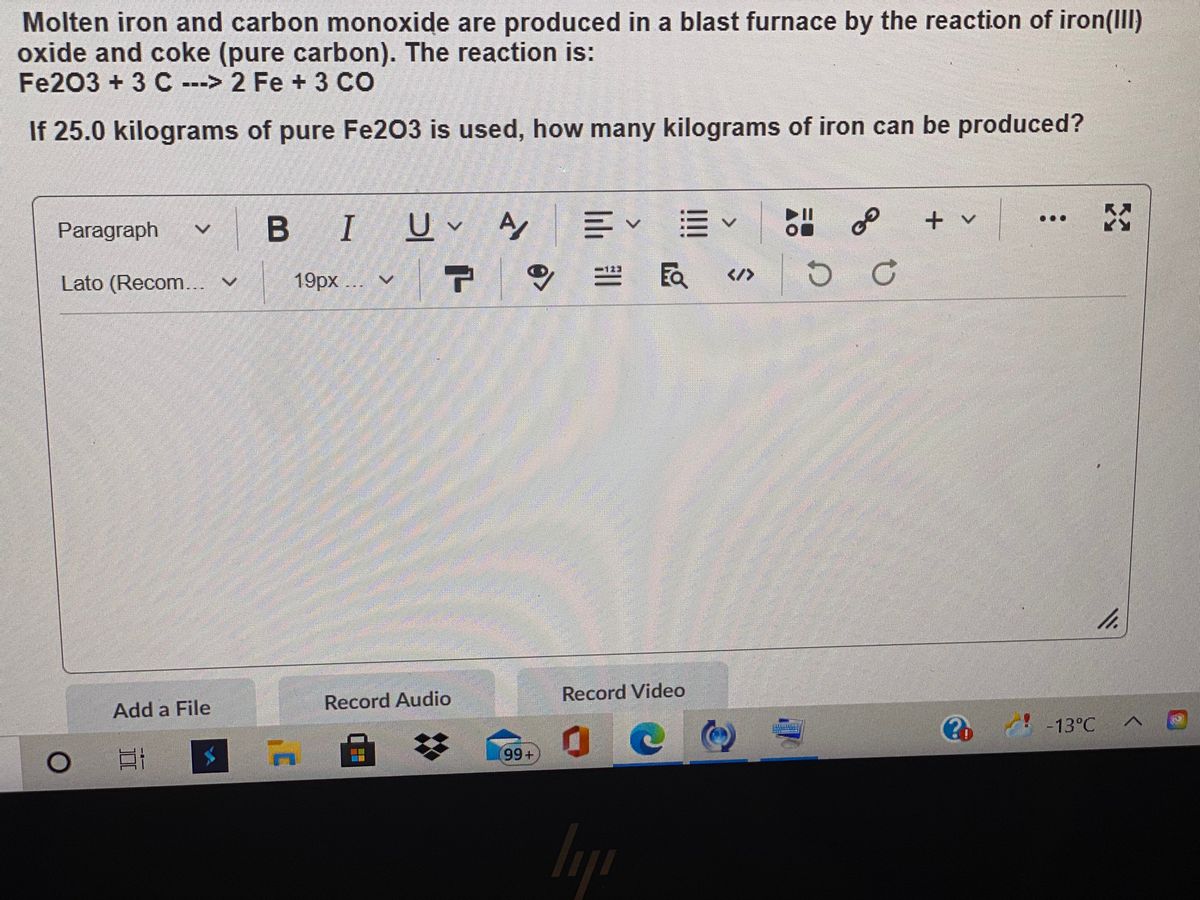
OneClass: 1. Iron is produced in a blast furnace from the reaction of iron( III) oxide with carbon...


![ANSWERED] Iron (III) oxide reacts with carbon monoxi... - Organic Chemistry ANSWERED] Iron (III) oxide reacts with carbon monoxi... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/41189624-1659104810.6526618.jpeg)